By: J.C. Arènes – Analyzer Specialist.
Like many nations, Canada has laid out ambitious plans to achieve net-zero greenhouse gas emissions by 2050, a goal that will undoubtedly rely on an increased reliance on clean hydrogen. While the world endeavors to move away from hydrocarbon fuel sources, the demand for hydrocarbons as building blocks for plastics and other materials remains, thus further compelling us to preserve this finite resource.
Already one of the top 10 hydrogen-producing nations in the world today[1], Canada is poised to benefit from its rich feedstocks and strong energy sector to become a major exporter of hydrogen and hydrogen technologies. Various processes exist already for the production of hydrogen, such as.
- Steam-methane reforming
- Biomass and coal gasification
- Electrolysis
Such is the interest in the new hydrogen economy and industry that there are now dedicated groups like the Canadian Hydrogen Working Group and Canadian Hydrogen and Fuel Cell Association on social media platforms like LinkedIn.
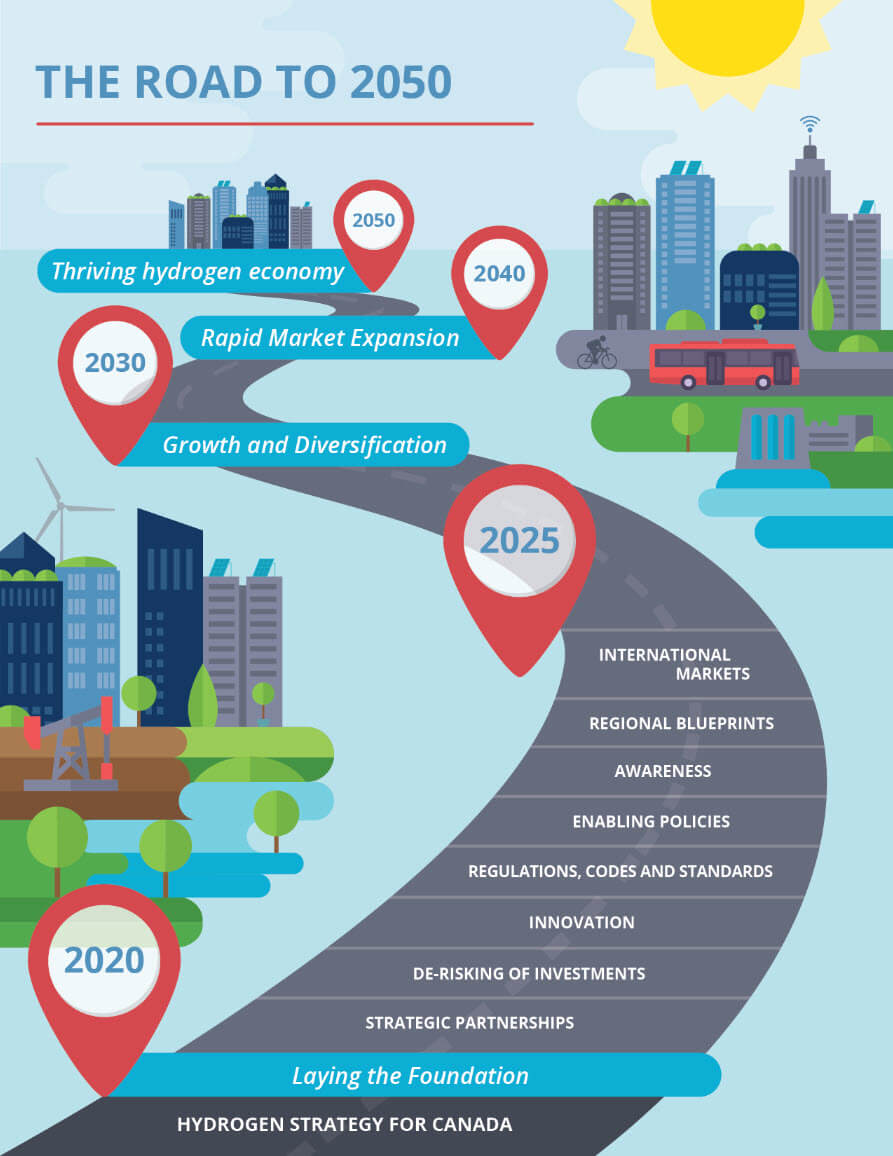
Regardless of the source or intended use for the hydrogen, reliable methods of analyzing hydrogen concentrations are a must for safety and process optimization. A number of analytical techniques exist in today’s market, each with its own advantages and challenges.
Solid State Analyzers
Solid state detectors take advantage of the hydrogen molecule’s tiny physical size to perform its measurement. Hydrogen molecules can easily migrate into the spaces within a Palladium-Nickel sensor matrix and impart changes to its electrical properties. The change in capacitance or resistance of the matrix can be correlated to a hydrogen concentration in a gas mixture, though there are some limitations in terms of physical conditions (temperature & pressure) and the sample’s composition.
Thermal Conductivity Analyzers
Thermal conductivity is a measure of a material’s ability to conduct heat. The thermal conductivity of hydrogen is significantly higher than any other gas, allowing us to use this physical property in its analysis. Measuring the thermal conductivity of a gas mixture can be used to ascertain its composition, though this technique is best applied to binary mixtures containing gases with dissimilar thermal conductivities.
Tunable Diode Laser Absorption Spectroscopy
It is well understood that the amount of energy absorbed by a gas at certain wavelengths (commonly in the infrared or ultraviolet spectrum) can be used as a technique to ascertain that gas’ concentration (according to the Beer-Lambert Law). The challenge with traditional photometers has always been in finding regions of the spectrum where interference from other gases in the mixture can be avoided (or minimized). Rather than using precision optical filters to manage the energy being monitored, tunable diode laser gas analyzers control the wavelength of the energy being created instead. This advanced technology has opened the possibility of measuring hydrogen gas concentration using infrared absorption spectroscopy. TDL absorption spectroscopy analyzers (TDLAS) also benefit from sometimes being suitable for installation directly on a process line or vessel without needing an extractive sample system.
Cavity Ring-Down Spectroscopy
A variation on TDLAS technology, Cavity Ring-Down Spectroscopy (CRDS), uses high precision reflective sample cells to achieve optical path lengths hundreds of kilometers in length. As per the aforementioned Beer-Lambert law, this allows for measurements at very low concentrations (often, parts per trillion). The time required for the energy of interest to decay as it travels hundreds of kilometers in the sample gas is a function of the component of interest’s concentration. In the case of hydrogen analysis in CRDS, the gas is first dried and then passes through a catalytic conversion module which changes the hydrogen molecules into easily measured water molecules.
Gas Chromatography
With the ability to physically separate complex gas mixtures into the components of interest, gas chromatographs (GC) are incredibly powerful analytical tools. In the case of hydrogen, once separated from the sample by the GC’s columns, its concentration can be measured by a simple thermal conductivity detector. The detector compares the thermal conductivity of the sample component with that of the constantly flowing carrier gas. GCs can be designed to measure multiple gas sample components, making them a popular choice in the chemical processing industry.
Mass Spectroscopy
Like the process gas chromatograph, a mass spectrometer performs a separation of the sample components in order to measure each individually. The sample is first ionized in a strong electrical field, and then the ions can be measured individually according to their mass/charge ratio. The software can then analyze the signals from the various mass/charge values and provide an analysis of the component concentrations. Mass spectrometers are often chosen in applications where their speed of response (usually only a few seconds) can be beneficial.
With such a dizzying array of analytical techniques, it might seem impossible to choose an analyzer for an application. However, every technique has its advantages and challenges. Consideration must be given to process conditions, the environment where the analyzer will be placed, analytical requirements, and of course, budget.
[1] NRCan.. (2020). Hydrogen Strategy for Canada, pp. I