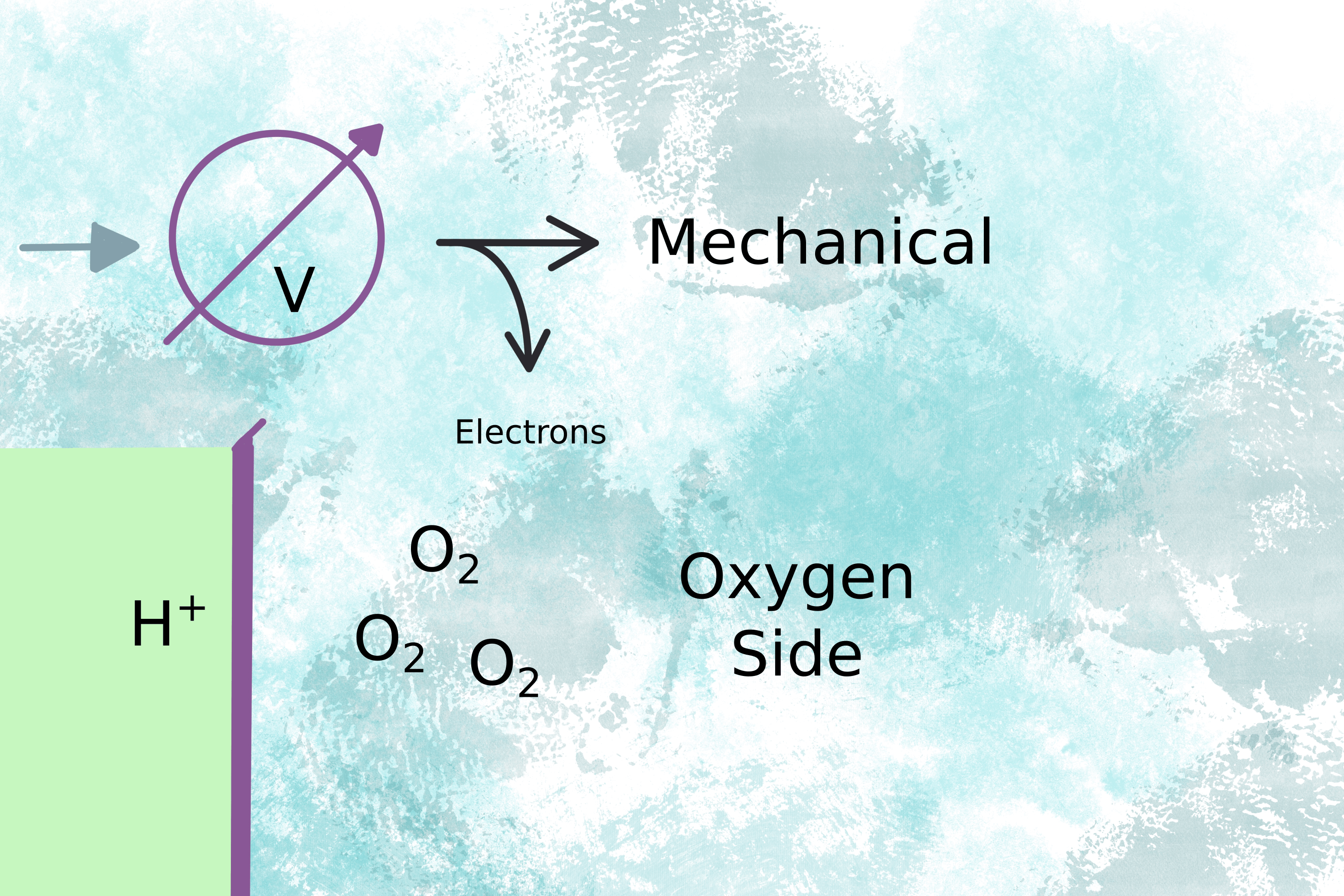Jim Kapron, PhD.
3 min read.
- Fuel cells calmly, quickly and efficiently turn hydrogen into energy and water
- Different types of fuel cells use different pressures of hydrogen
- The resulting water vapour may hinder hydrogen analysis
Fuel cells are part of Canada’s energy present. My current experience started in Sept 2020, shortly after I started at Novatech Analytical. A customer reached out with a problem: with all the other gases present, how do I know how much hydrogen has not yet been consumed by the fuel cell?
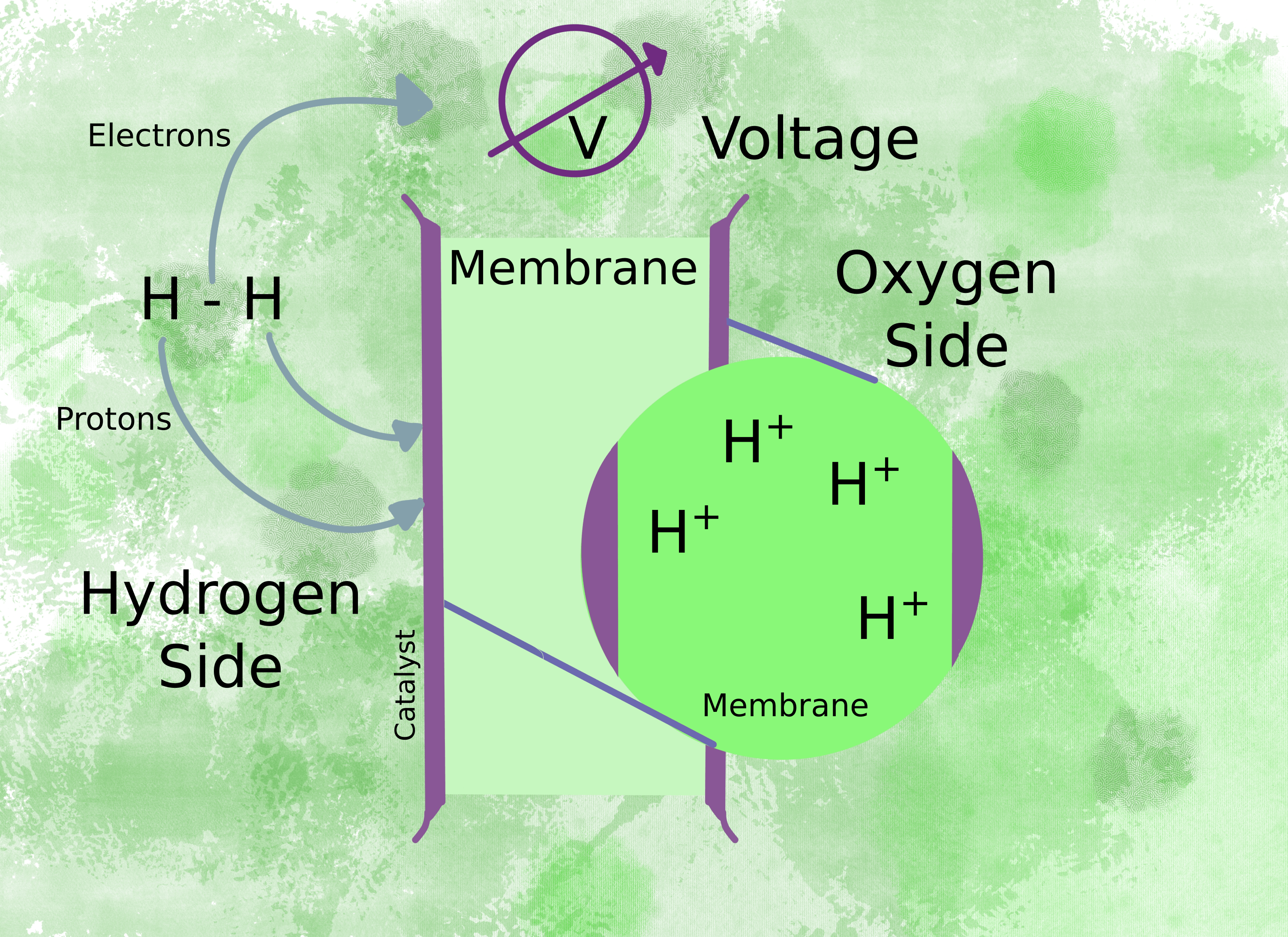
In this type of fuel cell, hydrogen ions pass through a membrane, and ideally, nothing else does. How does the starting material, hydrogen gas, change into the hydrogen ions that pass through? The membrane splits the hydrogen gas into two protons and two electrons. While the protons go through the membrane, the electrons go through a circuit to produce a voltage.
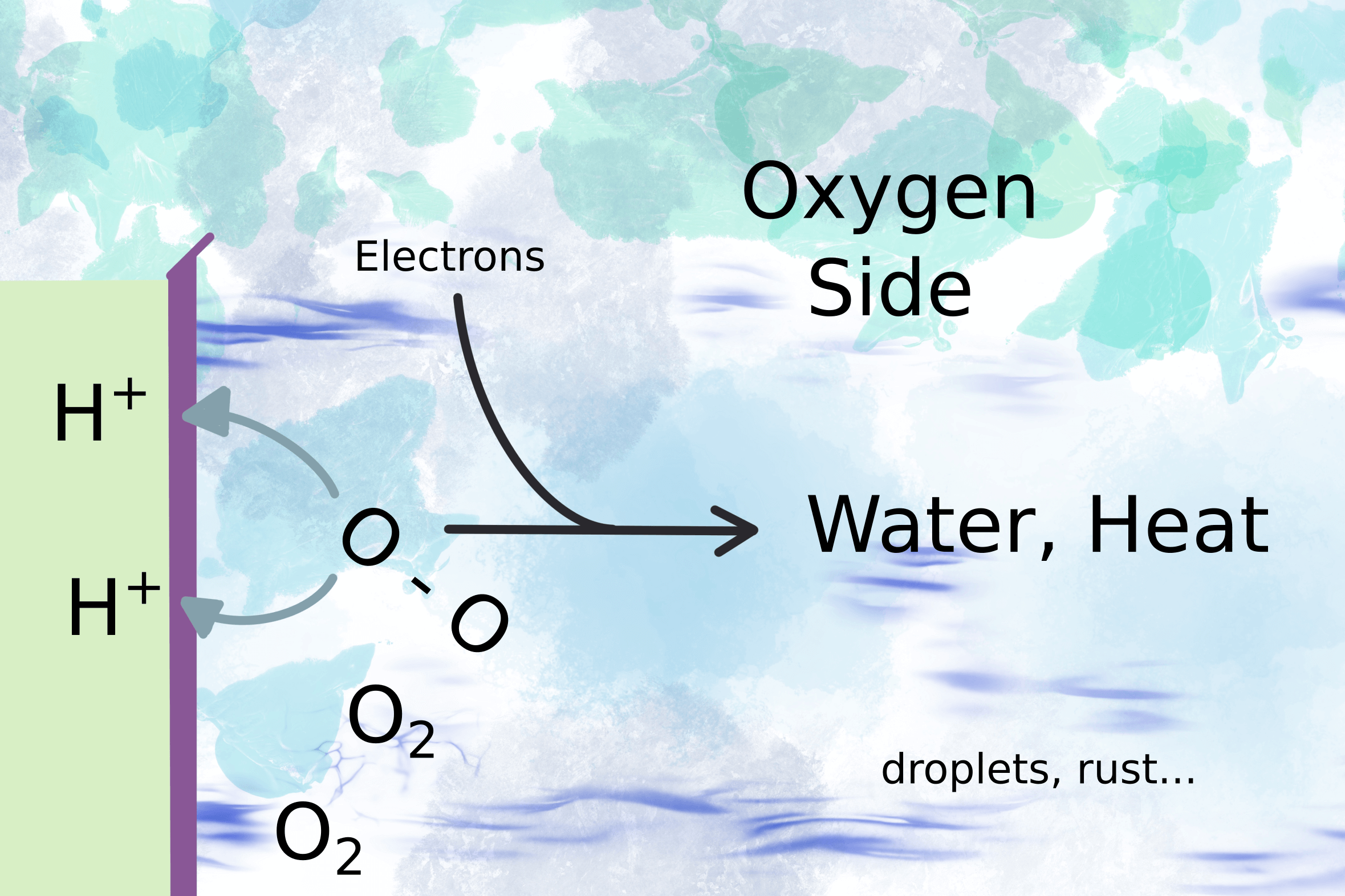
The voltage drives the motor (fig 2) and the electrons complete the circuit on the oxygen side of the membrane by combining with oxygen molecules to form water (fig 3).
Figure 2
Figure 3
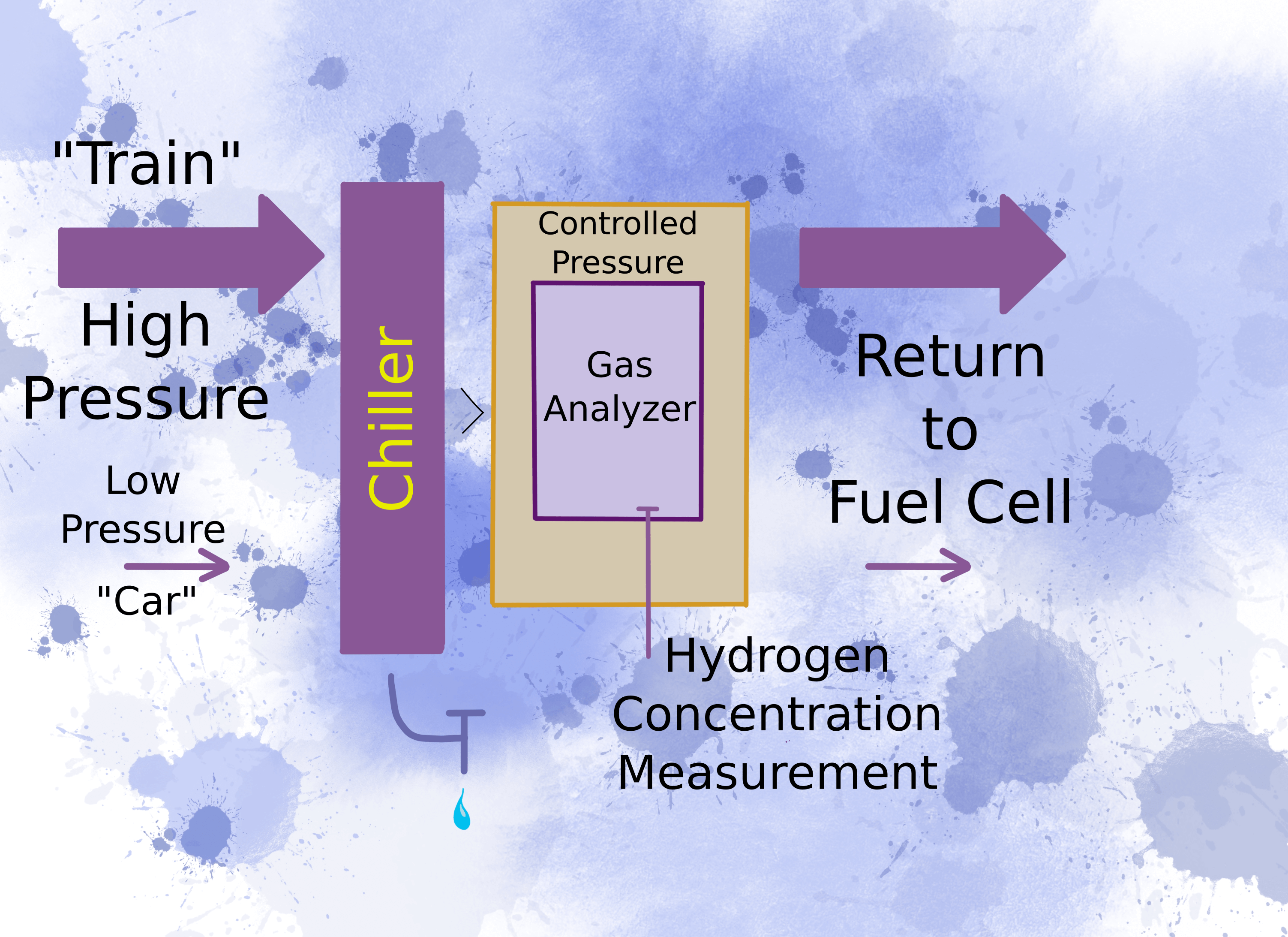
It sounds so simple, “water is formed”. But water droplets can stop a gas measurement immediately. Gas analyzers need to make sure the water stays in the vapour phase. Otherwise water droplets interfere with the gas measurement. So to measure the residual hydrogen gas, the first thing you need is a chiller to remove condensing water.
Different Pressures for Different Power
A passenger car needs much lower power than a three-kilometer long locomotive pulling this year’s wheat crop to the export terminal. You get these different power outputs from hydrogen pressure. Pressure relates to how many protons go through the membrane per unit time, which translates to power transmitted to the axle.
But how do you handle the varying pressure of different types of fuel cells? The trouble is that gas analyzers operate within a limited range of pressures. To resolve this apparent incompatibility, you need to raise the pressure above the highest expected fuel cell pressure to force the gas to the analyzer. Before it reaches the analyzer, you need to bring the pressure within the operating range of the analyzer and keep it constant during the analysis.
Once the hydrogen concentration is measured, force the gas back into the fuel cell so that none of it is wasted.
Remember when I said that nothing but hydrogen should pass through the membrane? Well, what if the oxygen passes through to the hydrogen side? That’s a real concern and best addressed in a future blog post.
Summary: Canada has the resources, world-leading companies, and intelligent workers to bring energy balance into reality. Future blog posts will discuss storage, transport, and consumption of hydrogen, plus how to recuperate the resulting products. As for my first experiment, where the oxygen actually went is an entirely different story of detective work for another blog post.
If you would like to know more about analyzing hydrogen or its impurities, in gas or liquid form, give Novatech a shout.
About the author: Jim is a real-world scientist helping to move the BC economy forward with gas and liquid analyzers. In his off hours, he’s involved with music and video production. Ask him a question, and he’ll locate resources to find an answer.