Jim Kapron, PhD.
3 min read.
- Electrolysis of fresh water is preferred, but it’s a scarce resource.
- Electrolysis of sea water needs stringent conditions and new techniques.
- When oxygen is produced, it can still slip across the membrane resulting in potentially unsafe conditions.
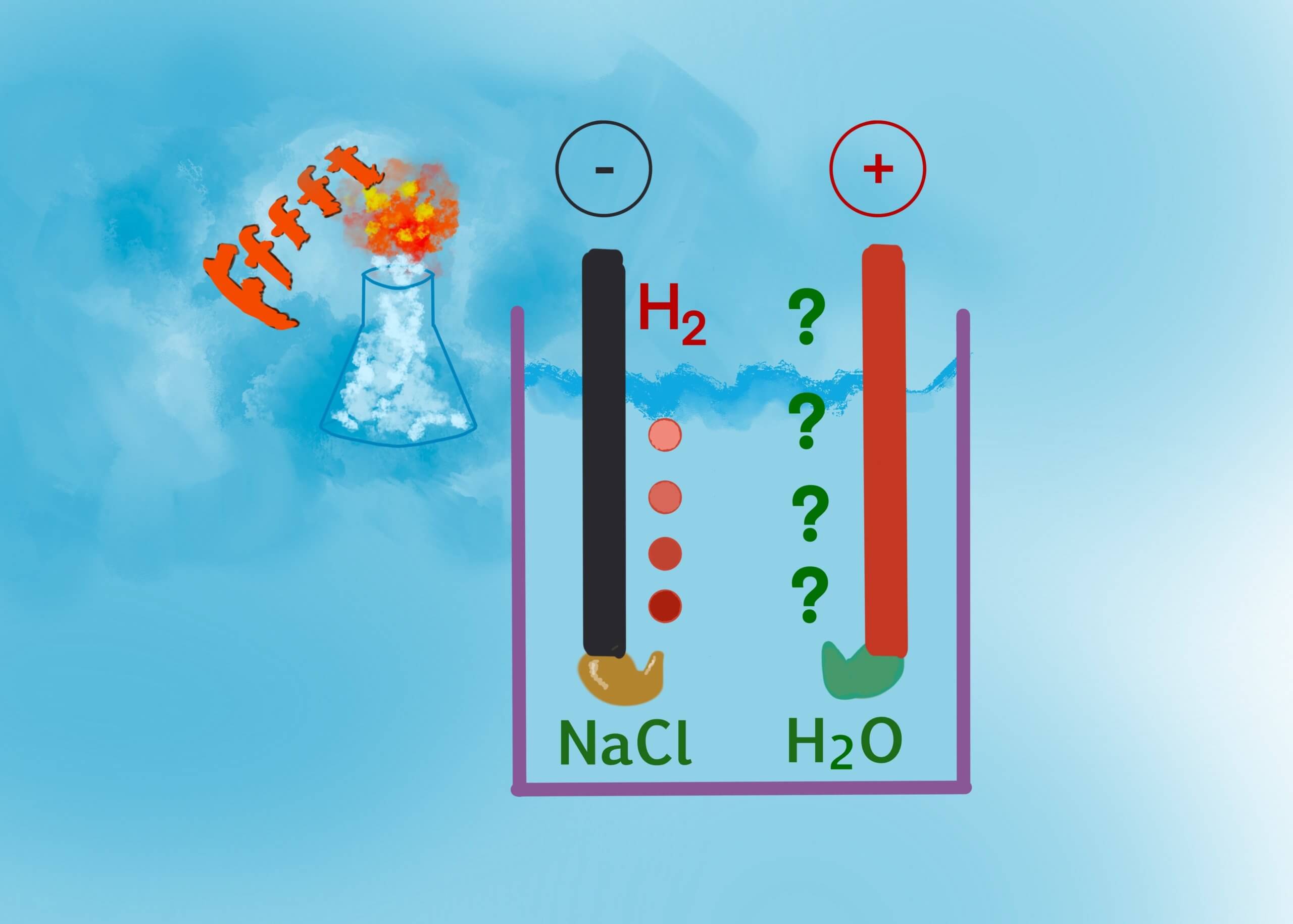
I first made hydrogen in my early teens, but I never did make any oxygen (see blog post 1). It perplexed me because textbooks say water is made of hydrogen & oxygen. Instead, I made a dull green paste on the surface of my copper electrode (Figure 1). Recently, I learned I’m not the only one to have difficulty making oxygen. Electrolyzing sea water turns out to have some challenges.
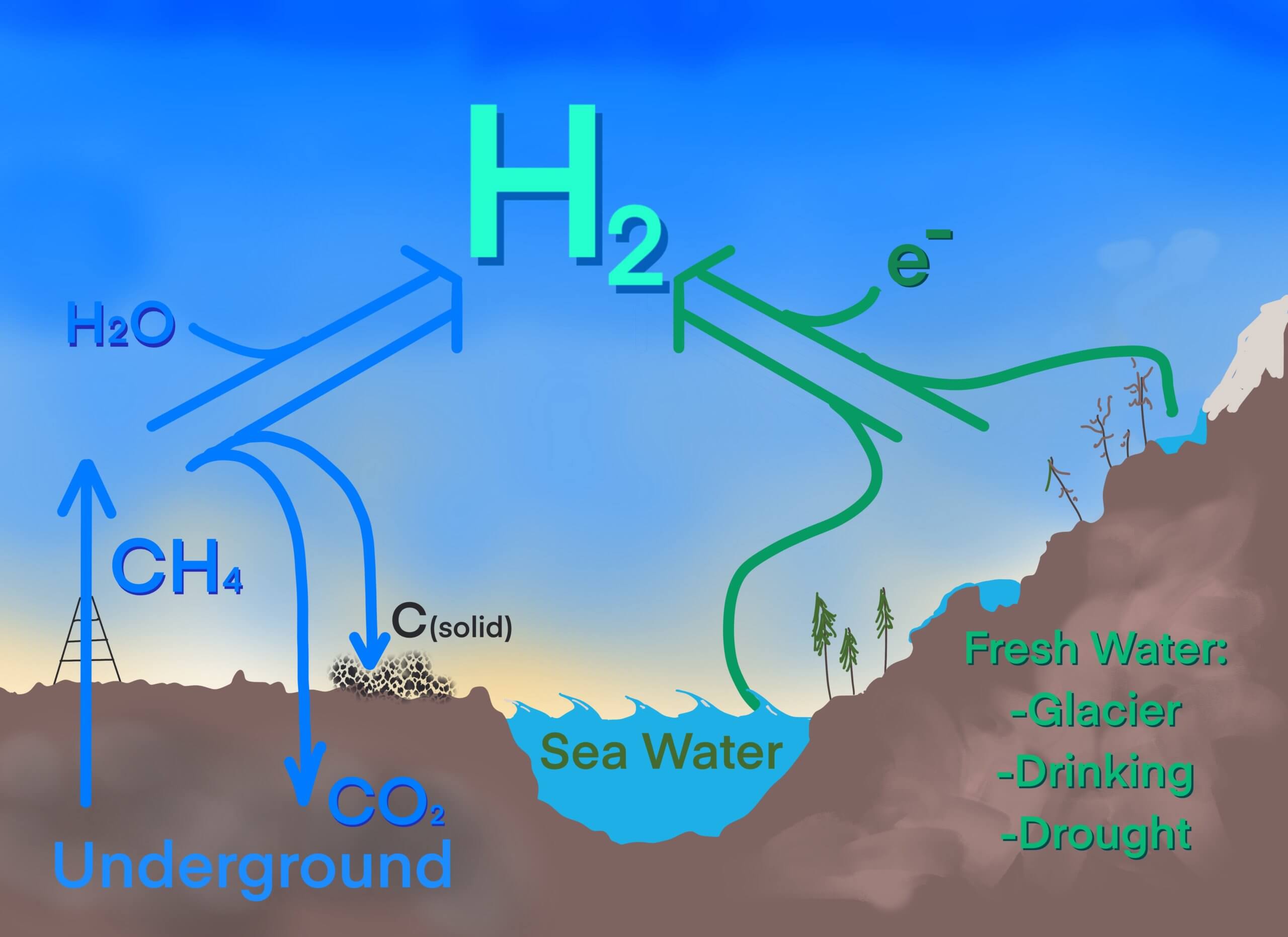
We have debates at Novatech: to make the massive amounts of hydrogen our society needs, some think natural gas is the only way to go via some variation of steam-methane-reforming (Figure 2). This could involve either capture & storage of carbon dioxide or new, creative technologies that generate hydrogen and solid carbon. But read any news article: green hydrogen – via the electrolysis of fresh water – is the preferred route.
We’re very lucky in Canada because we have more than our fair share of drinking water in the great lakes. Yet, this past summer parts of our rain forest, for the first time ever, imposed water restrictions on corporations. The prairie provinces know about drought. Our glaciers are melting. Sea levels are rising. Tuktoyaktuk is sinking. Drinking water is more precious than it’s ever been.
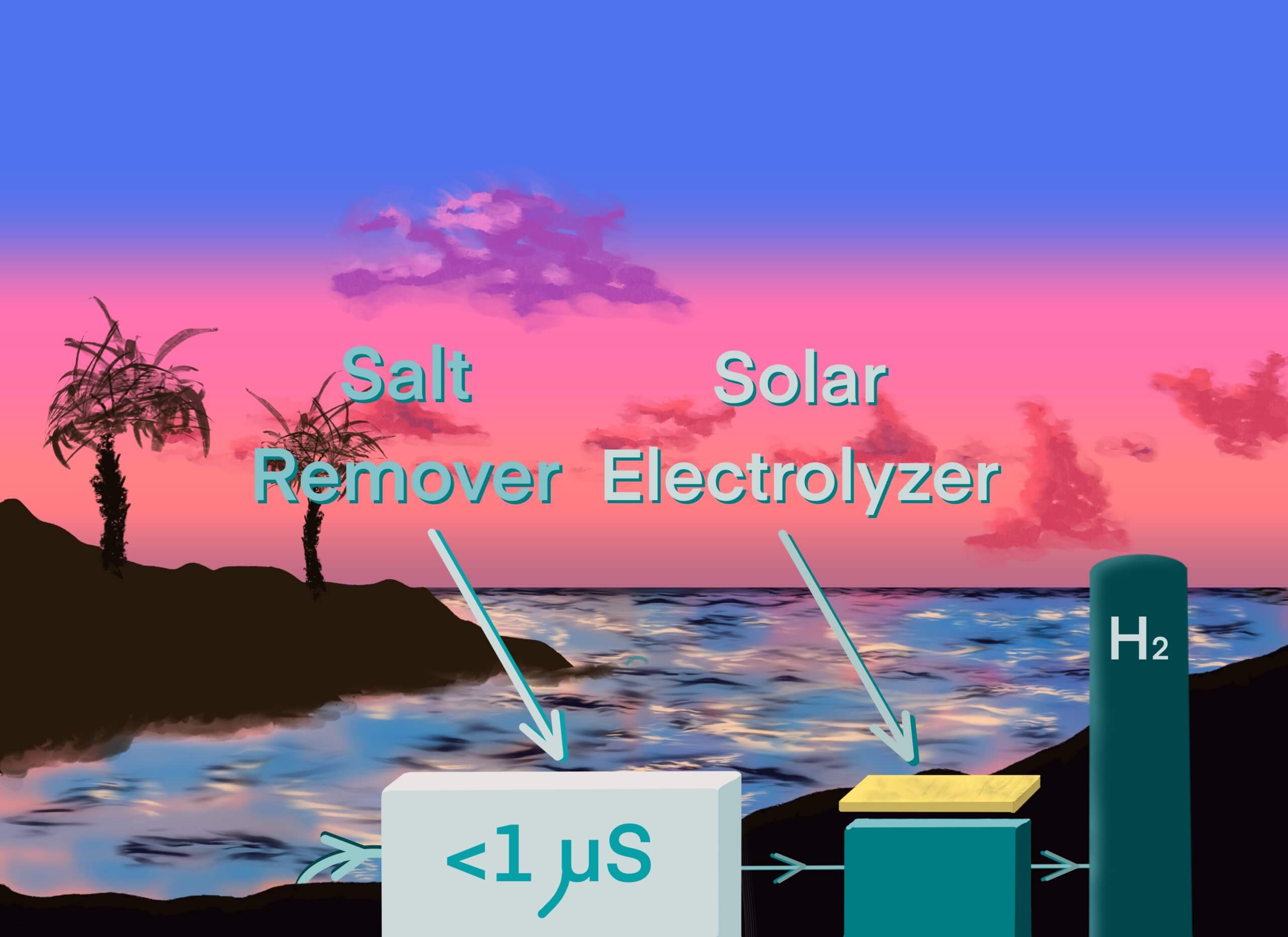
A recent article from Portugal showed a body of water, the water purification system, and a solar-powered system to generate hydrogen. My interpretation is in Figure 3. The water purification system only indicates “<1µS”, which suggests a reverse osmosis system, similar to the systems in the parched areas of the middle east. It sounds so simple – just remove the salt. The reality is energy-intensive and far from simple.
Canada is not graced with the same year-round sunshine of the middle east or even Portugal, for that matter. We’ll probably end up with a mixture of solar and wind to power many of our future electrolyzers, but not exclusively. Not everywhere has hydro-electric dams. But does it make sense to remove the salt from sea water before electrolysis?
At normal current densities like I used in my first experiment, hydrogen and chlorine are formed. That’s very useful to make chlorinated compounds, but they have their own risks. At either low or high current densities, you *can* make oxygen. There is still much work to be done on selective membranes, catalysts, and pH control of the membranes to make this economical.
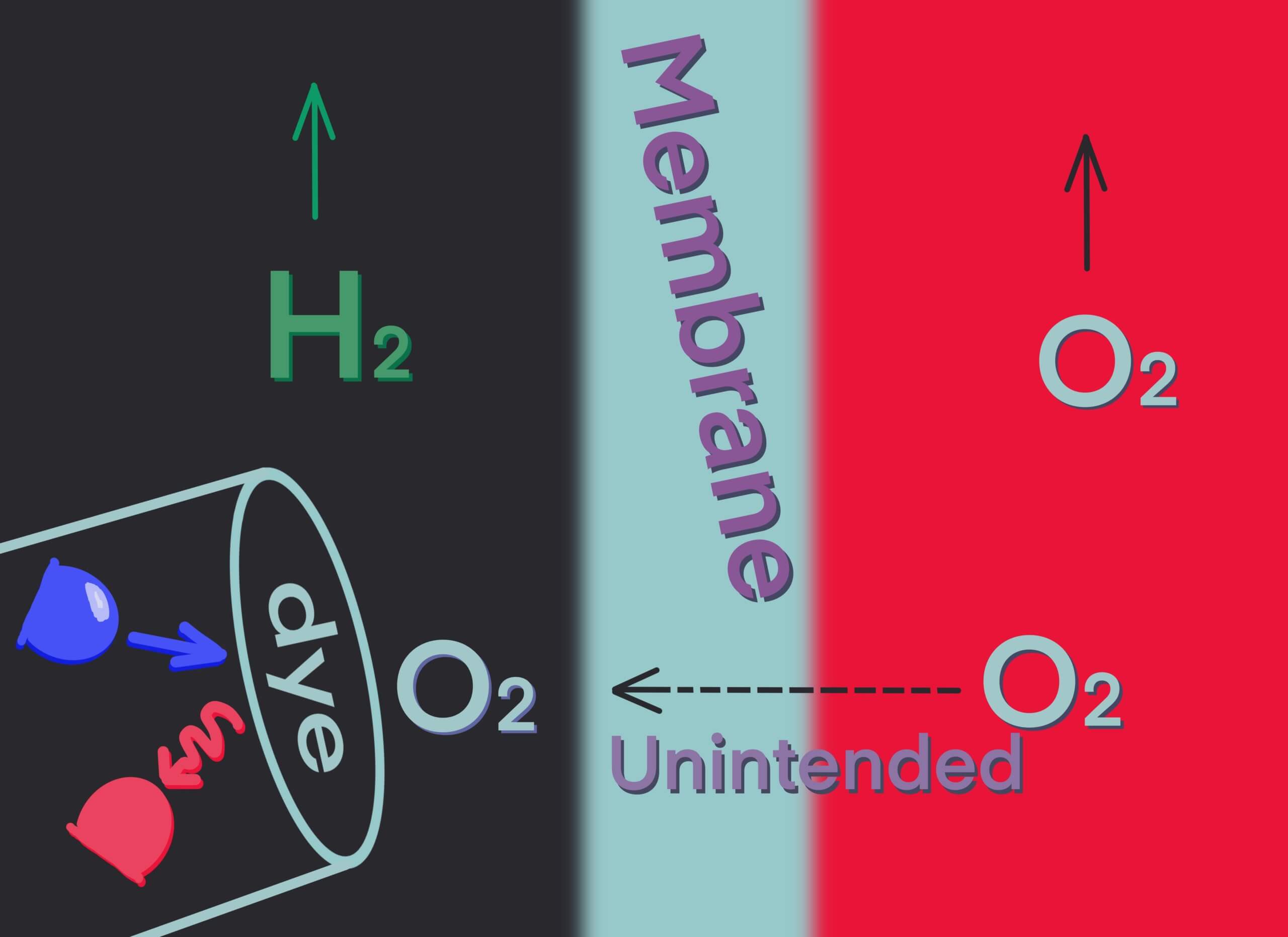
Even if you’re successful at making oxygen at the anode (see blog post 2), oxygen molecules can also slip across the membrane to the hydrogen side with potentially disastrous effects. Which brings me to this interesting little oxygen analyzer (Figure 4).
A dye impregnated on the membrane will fluoresce if oxygen is not present. An LED shines light on the membrane, and the photocell registers the fluorescence. If oxygen is present, the fluorescence is quenched, proportional to the oxygen concentration. It works in vacuum, in the gas phase, or in liquid. Any oxygen crossing the membrane can tell the operator to change the membrane, adjust the process, or even shut the electrolyzer down, which is a good reason to know where the oxygen is.
Oh, and that dull green precipitate was CuCl2. The copper electrode was consumed by the chloride ions in solution. I got the explosion I wanted, but the oxygen went into forming NaOH, raising the pH, and reducing the reaction rate, making hydrogen production less efficient.
That’s where the oxygen went and another reason why you should care where it goes.
Summary: Now that I’ve shown you where the oxygen went, the next blog entries will focus on the storage, transport, and consumption of hydrogen and what to do with all this water.
If you would like to know more about analyzing hydrogen, its impurities, by-products, in gas or liquid form
About the author: Jim is a real-world scientist helping to move the BC economy forward with gas and liquid analyzers. In his off hours, he’s involved with music and video production. Ask him a question, and he’ll locate resources to find an answer.